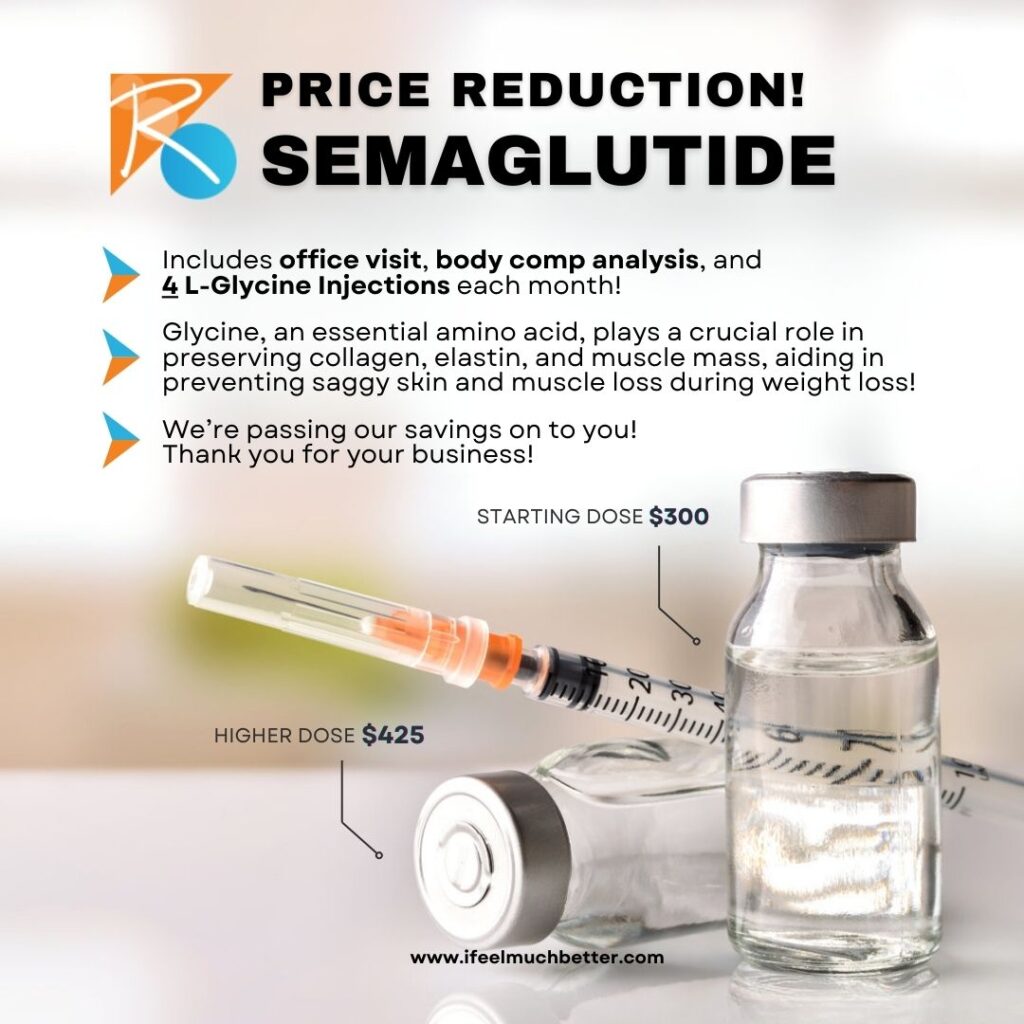
Dive into a transformative journey with our all-inclusive Semaglutide regimen, meticulously designed to invigorate your body and preserve your vitality! Experience the ultimate blend of wellness and savings with our new offer that includes your office visits, comprehensive body composition analysis, and 4 potent L-Glycine injections every month!
Glycine, the unsung hero among amino acids, takes center stage, boasting unparalleled benefits for your body. It works tirelessly to safeguard your collagen, elastin, and precious muscle mass, safeguarding you against the dreaded effects of weight loss – saggy skin and muscle depletion.
And that’s not all – we’re thrilled to pass on our savings directly to you! Embrace this opportunity to prioritize your well-being without breaking the bank. With a starting dose of just $300, or opt for a higher dose at $425, every penny invested promises priceless returns in terms of health and confidence.

med spa in Kansas City
Functional Medicine + Aesthetics
At Regain Functional Medicine + Aesthetics, transformation is what we do. We’re a functional medicine clinic and full-service med spa in Kansas City that exists to help restore your health, enhance your appearance, and rebuild your confidence.
Unlike many wellness centers and med spas, we treat the whole person from the inside out. Our dedicated providers take the time to get to know each patient and build long-term relationships around achieving and maintaining transformational results.
Customized Care
We create personalized treatment plans to support the unique needs and goals of each of our patients.
Functional Health
Instead of focusing on individual symptoms, we treat the whole patient from the inside out.
Aesthetic Services
As a full-service med spa, we use the latest in aesthetic treatment technology to help you look your best.
Introducing!
Upcoming Events!
Welcome Video!
Watch this video to dive in, and get a deeper understanding about Regain Functional Medicine & Aesthetics.
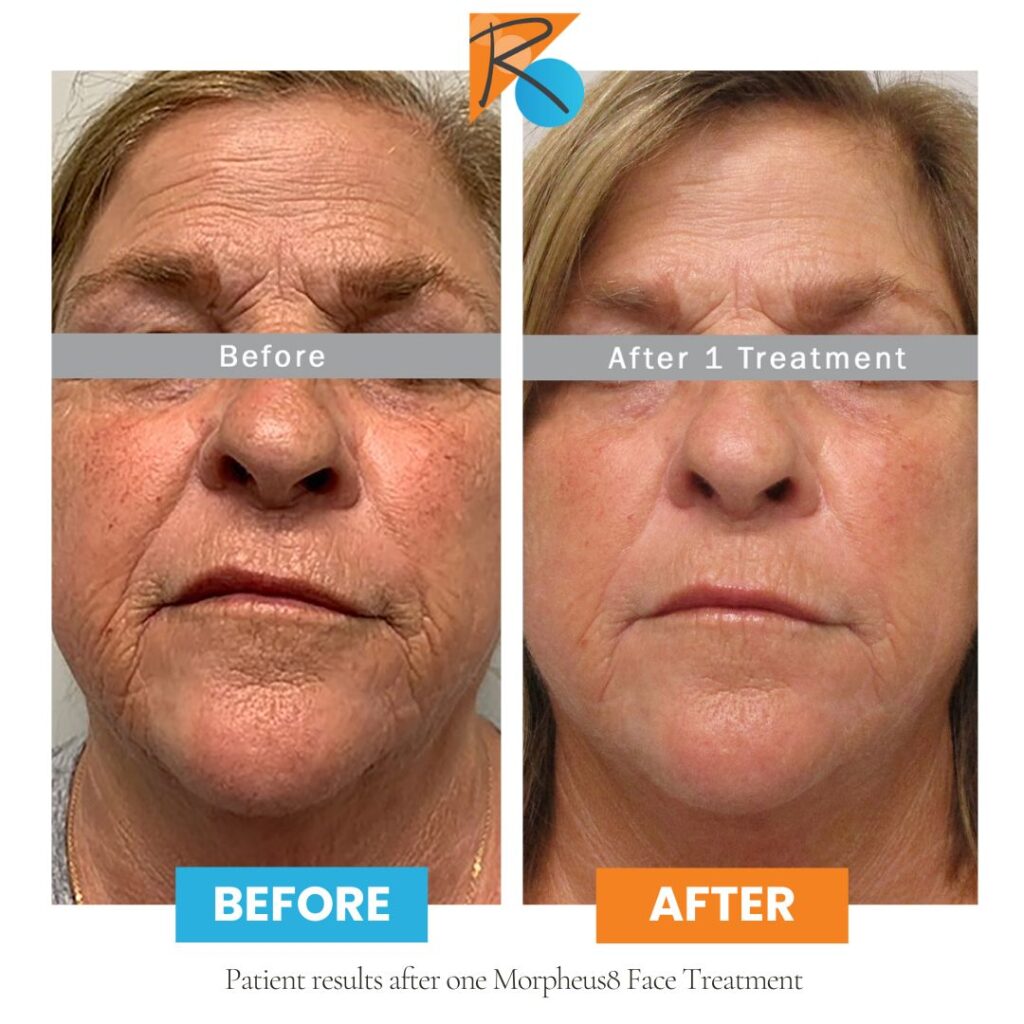
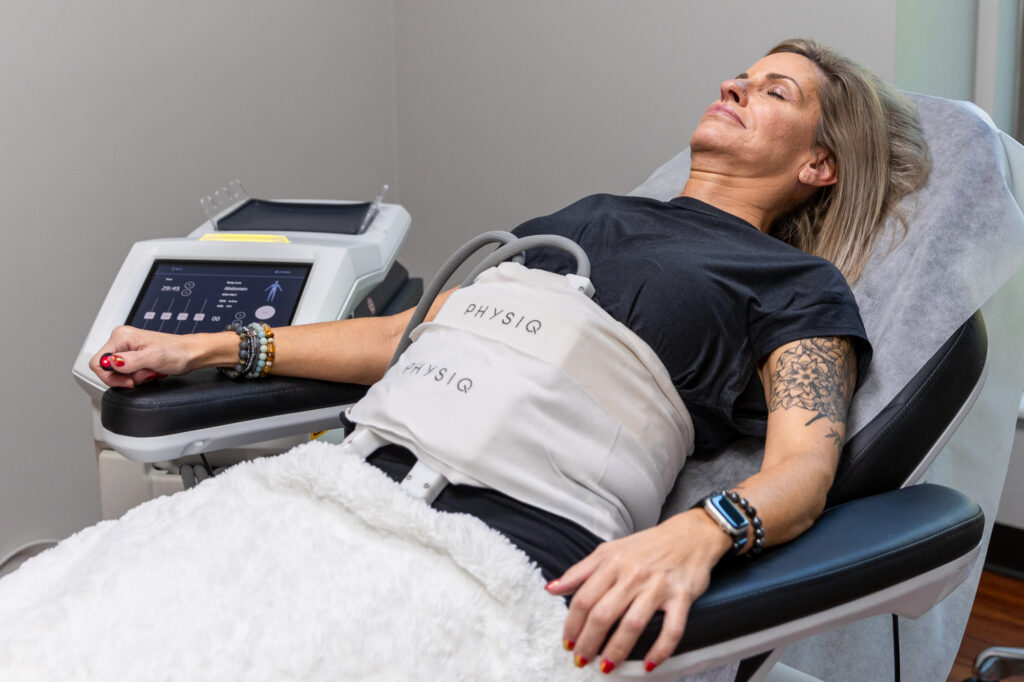
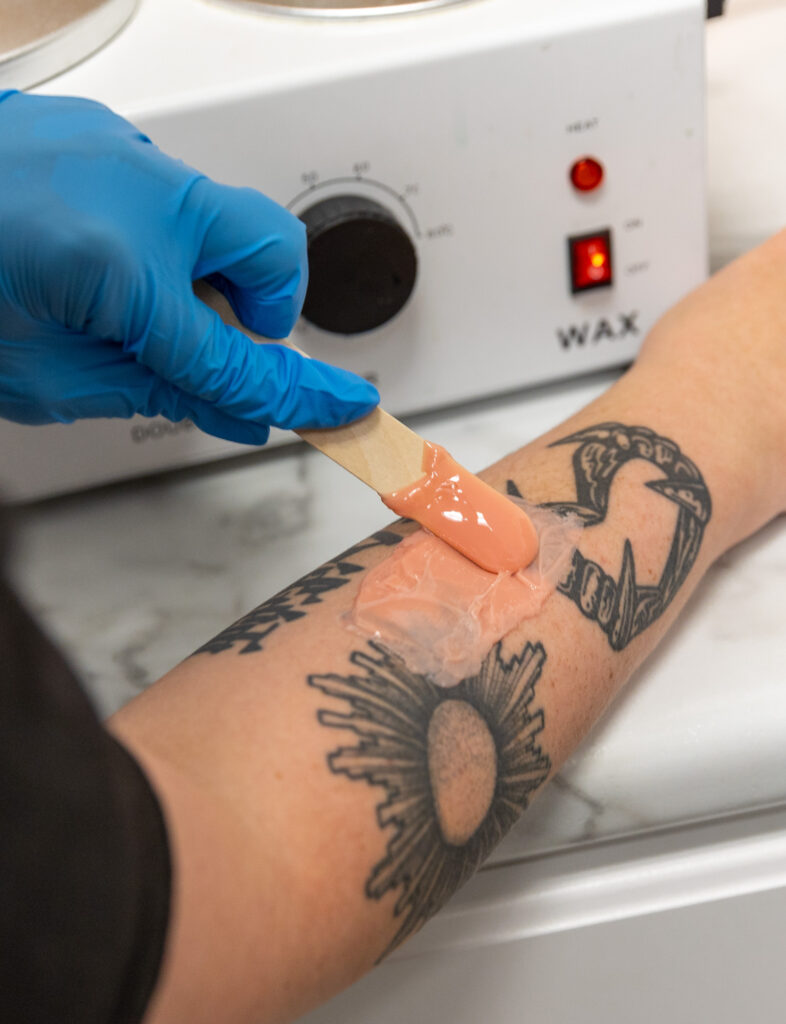
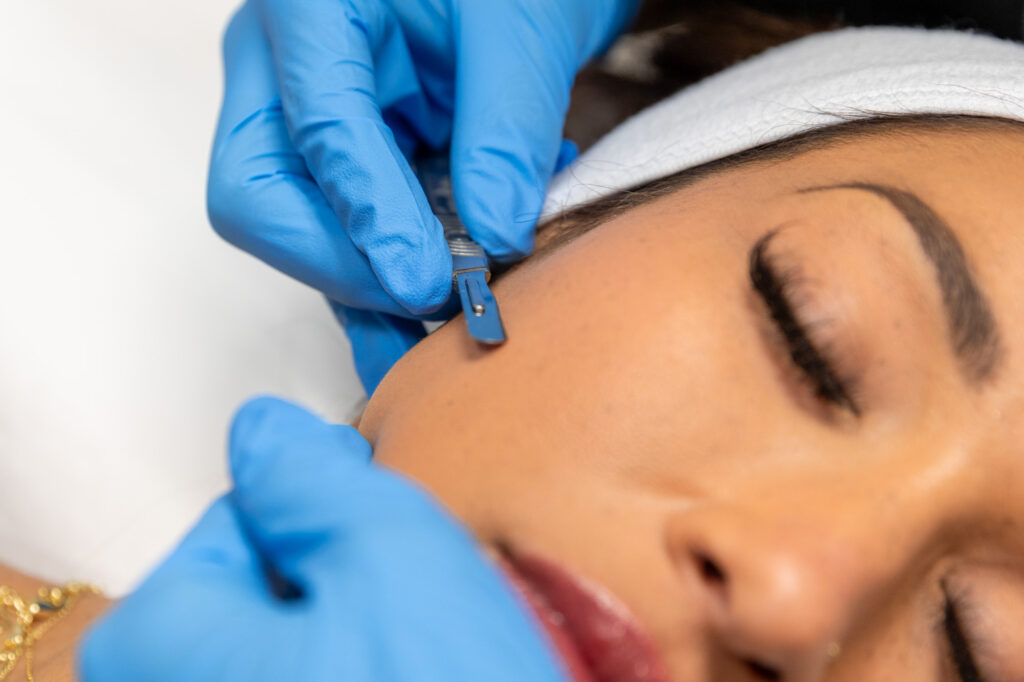
Popular Treatments
Take a look at some of the popular treatments for Regain Functional Medicine, a med spa in Kansas City.
Our Specials

Get Started
Use our Treatment Planning Tool to tell us more about your unique needs and goals. We’ll turn your input into personalized treatment recommendations for getting you to where you need and want to be.
Tuesday: 9:00am - 5:00pm
Wednesday: 9:30am - 6:00pm
Thursday: 9:00am - 5:00pm
Friday: 9:00am - 2:00pm
Saturday & Sunday: Closed
Tuesday: 9:00am - 5:00pm
Wednesday: Closed
Thursday: 9:00am - 5:00pm
Friday: Closed
Saturday & Sunday: Closed
Feel Better •
Function Better •
Move Better •
Look Better •
Feel Better •
Function Better •
Move Better •
Look Better •

Your Better Life Starts at Regain
Start your complimentary consultation with our experts today.